On 30th June 2024 the third CANDY project period has officially ended. This means one year to go and the CANYD consortium had to submit a detailed progress report to our funding organisation, the European Commission. Although we encounter a slight delay in our clinical studies, PIP-CANDY and Multiplex (MPX), pace has been accelerated over the last months and especially the pre-clinical working groups are close to finish their tasks.
Please see here our Publishable Summary of this Periodic Report.
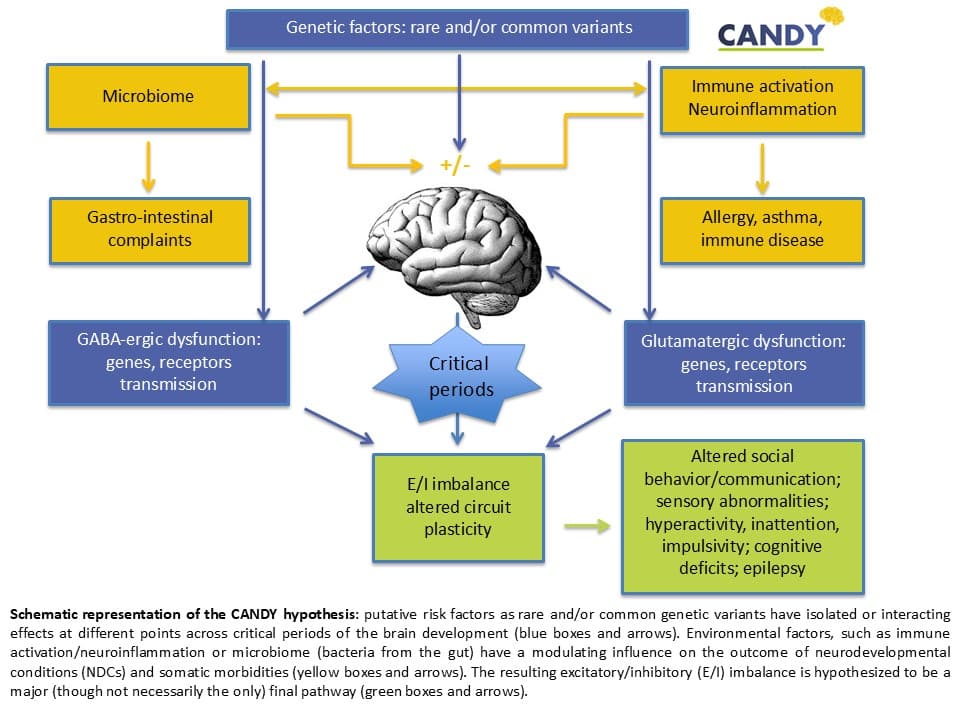
Summary of the context and overall objectives of the project
Between 50 to 75 million children and adults in Europe present with neurodevelopmental conditions (NDCs) that arise early in life. These conditions affect the development of the brain. This may lead to abnormal brain structure and function that is associated with impairments of social behavior and communication, emotion, learning ability, and self-control. These conditions include autism, attention-deficit hyperactivity disorder (ADHD), Intellectual Disability (ID), and language disorders, and often pose a burden for these individuals and their families, and for society. This burden is due to the high prevalence, the lasting effects for a person’s lifetime, the overlap of these conditions with each other, and the frequent combination with somatic conditions such as epilepsy, allergies, immune and gastrointestinal diseases. Persons with these conditions live on average 20 years shorter than individuals in the general population. The main problem is that there are no effective interventions for these conditions, and that our understanding of the underpinning pathophysiology is fragmentary. It was known that genetic factors play an important role in these conditions. The goal of CANDY is to improve our understanding of how the immune system, and the bacteria in the gut (microbiome) impact on these genetic factors. This will provide a novel conceptual framework to understand the pathophysiology of these conditions. The specific aims are 1) Elucidate the causal mechanisms that underlie these conditions and the combination with somatic conditions, 2) Deliver novel biomarkers to guide early diagnosis and prevention, stratification and/or treatment monitoring, 3) Lay the groundwork for new strategies for prevention and treatment of neurodevelopmental conditions and epilepsy, and 4) Open-up new avenues for research.
Work performed from the beginning of the project to the end of the period covered by the report and main results achieved so far
We have assessed sensory processing, behavioural phenotypes and electroencephalogram (EEG) recordings in two genetic autism mouse models at various development stages and performed behavioural profiling of another mouse model for autism and ADHD. The autism models reveal different sensory information processing deficits, which provides a basis to establish readouts for pathogenic pathways. Sensory hyperexcitability in one of the models is associated with increased wakefulness that may be linked to disturbed circadian systems. In one model, we induced inflammation during pregnancy and found that this moderated the influence of genetic vulnerability on autism symptoms.
Our work on a translational test battery for mice demonstrates that genetically modified mice show increased anxiety-like behaviour and changes in the expression of two glutamate receptor subunits in the striatum in adulthood. We have created a new set-up that combines physiological measures with behaviour in freely moving animals in complex behavioural tasks. Our related work on a tablet test battery for preschoolers demonstrates initial external validation of a novel executive function and reward learning task against clinical features and fronto-parietal anatomical differences on Magnetic Resonance Imaging (MRI) scans.
We identified a possible genetic interplay between gut inflammatory processes and autism. This has led to the inclusion of additional markers such as gut-brain markers, brain autoantibodies, and gut autoantibodies in our set of immune profiling markers. A meta-analysis compared the gut microbiota landscape between adults with and without ADHD and found specific types of bacteria to be associated with ADHD.
The protocol for the preschool imaging project (PIP) in CANDY has obtained ethics approval at all study sites, and we created standard operating procedures for data collection of these measures and trained researchers on relevant clinical instruments. We could catch-up recruitment and have reached 84% of the PIP recruitment target. Quality control and preprocessing have started and working groups have formed to establish data-analytic and publication strategies for each data domain and across-domains. We successfully evaluated the efficacy of the novel artificial intelligence tool that employs a convolutional neural network to enhance scan quality, effectively correcting motion artifacts within PIP preschool MRI scans.
The protocol for our clinical study in families with two or more individuals with an NDC (Multiplex study) has been approved for all study sites, and we have reached the recruitment target of 104 Multiplex families. We completed the whole genome sequencing for 60 families while the sequencing for the remaining 40 families is ongoing. The pipelines for variant calling and validation are ready. We made an operative list of genes associated with NDCs and conducted a large-scale study on more than 13,000 individuals with autism and 210,000 undiagnosed individuals. We were able to provide a gene-level map of the prevalence of loss of function variants affecting 220 genes robustly associated with autism.
We have finalised the consortium-wide database and delivered multiple new statistical techniques as implementable and usable tools.
Progress beyond the state of the art, expected results until the end of the project and potential impacts
The intended and expected impact of CANDY is to give new directions for clinical research to improve prevention, diagnosis, prognosis, treatment, and management of neurodevelopmental conditions and their association with somatic conditions. Research in CANDY will lay the groundwork for prevention of early maternal immune-activation, for new intervention strategies (immune-modulation/stem cells, or probiotics, nutrition; and/or identify new druggable targets) for neurodevelopmental conditions (autism, ADHD, Intellectual Disability, and epilepsy). We will identify developmental windows of opportunity for these interventions, and provide clinical recommendations for (early) diagnosis, prevention, and treatment of NDCs and associated somatic conditions. Whenever relevant, identified biomarkers can be used for more accurate and earlier diagnosis, prognosis as well as monitoring of patients’ condition. We will deliver and test biomarkers (genetic/immunogenetic, immune-related, microbiome, eye-tracking, neurocognition and neuroimaging) for (early) diagnostic, predictive, mechanistic and stratification purposes. Also, we will build prediction models for risk and onset of epilepsy (using EEG measures) or gastro-intestinal or (auto)immune diseases in persons with neurodevelopmental conditions from preschool to adult age.